Silexion Therapeutics Announces New Preclinical Data Showing Up to 97% Inhibition of Cancer Cell Growth, Including New Evidence Against New Previously Untested KRAS Mutation
New Results Reveal Unprecedented Inhibition Rates and First Evidence of SIL204’s Efficacy Against Previously Untested KRAS Q61H Mutation in Human Cancer Cells, Significantly Expanding its Pan-KRAS Potential; Company on track for the initiation of a Phase 2/3 clinical trial in Q2 2026
Grand Cayman, July 31, 2025 (GLOBE NEWSWIRE) — Silexion Therapeutics Corp. (NASDAQ: SLXN) (“Silexion” or the “Company”), a clinical-stage biotechnology company pioneering RNA interference (RNAi) therapies for KRAS-driven cancers, today announced groundbreaking new preclinical data revealing unprecedented inhibition rates of up to 97% in pancreatic cancer cells and almost 90% in colorectal cancer cells. These findings, which include the Company’s first evidence of efficacy against the clinically significant KRAS Q61H mutation in human cancer cells, represent a substantial advancement from previously reported results and further establish SIL204’s potential as a pan-KRAS therapeutic.
While the Company has previously reported activity in various cancer models, these latest findings from a comprehensive CTG (Cell Titer-Glo) analysis demonstrate significantly higher inhibition rates and provide the first direct comparison of SIL204’s potency across multiple cancer types and KRAS mutations in a single study, including dose-dependent inhibition of up to 94% in pancreatic cancer cells with KRAS G12D mutations, comparable 97% inhibition in pancreatic cancer cells with the previously untested KRAS Q61H mutation.
Key findings from the preclinical studies include:
- SIL204 demonstrated dose-dependent inhibition of up to 94% in pancreatic cancer cells harboring KRAS G12D mutations at nanomolar concentrations
- SIL204 showed comparable efficacy of approximately 97% inhibition in pancreatic cancer cells with KRAS Q61H mutations, a variant not previously reported in the Company’s studies
- SIL204 produced an inhibition rate of nearly 90% in colorectal cancer cells with KRAS G12D mutations, extending previous evidence of its effectiveness beyond pancreatic cancer
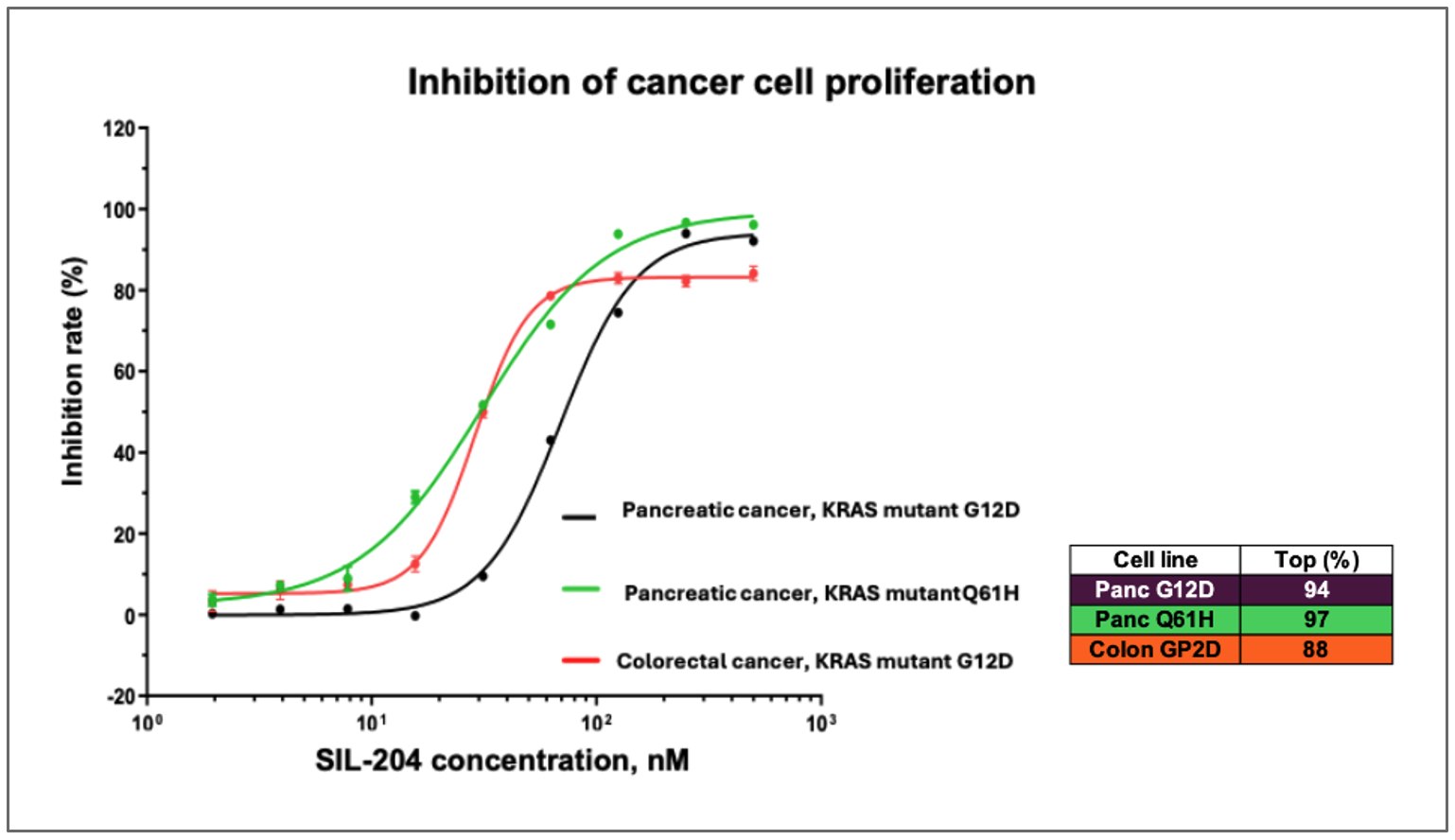
Figure: SIL204 demonstrates dose-dependent inhibition of cancer cell proliferation across multiple tumor types and KRAS mutations, including pancreatic cancer with KRAS G12D (purple line) and Q61H mutations (turquoise line), as well as colorectal cancer with KRAS G12D mutation (orange line).
“These findings provide compelling evidence of SIL204’s potent activity against multiple KRAS mutations across different cancer types,” said Ilan Hadar, Chairman and Chief Executive Officer of Silexion. “The ability to achieve such high levels of inhibition in both pancreatic and colorectal cancer models with different KRAS mutations substantially strengthens SIL204’s potential as a pan-KRAS therapeutic candidate. With these promising results across all three major KRAS-driven cancer types – pancreatic, colorectal, and lung – we’re increasingly confident in SIL204’s potential to address significant unmet needs for patients with these aggressive cancers.”
This announcement comes just days after Silexion reported significant efficacy of SIL204 in lung cancer cell lines, validating the Company’s innovative lipid-conjugated delivery system. Together, these results across pancreatic, colorectal, and lung cancer models provide comprehensive evidence of SIL204’s broad applicability against KRAS-driven cancers.
“With our data now covering pancreatic, colorectal, and lung cancer models, we’ve demonstrated SIL204’s consistent efficacy across the three most common KRAS-driven cancer types, added Ilan Hadar. Particularly notable is SIL204’s high efficacy against the KRAS Q61H mutation, which expands our understanding of its potential against multiple KRAS variants.”
Silexion continues to prepare for the initiation of a Phase 2/3 clinical trial in Q2 2026 to investigate SIL204 for the treatment of KRAS-driven solid tumor cancers, leveraging both intratumoral and systemic delivery approaches as part of the Company’s dual-route administration strategy.
About Silexion Therapeutics
Silexion Therapeutics is a pioneering clinical-stage, oncology-focused biotechnology company developing innovative RNA interference (RNAi) therapies to treat solid tumors driven by KRAS mutations, the most common oncogenic driver in human cancers. The Company’s first-generation product, LODER™, has shown promising results in a Phase 2 trial for non-resectable pancreatic cancer. Silexion is also advancing its next-generation siRNA candidate, SIL204, designed to target a broader range of KRAS mutations and showing significant potential in preclinical studies. The Company remains committed to pushing the boundaries of therapeutic innovation in oncology, with a focus on improving outcomes for patients with difficult-to-treat cancers. For more information please visit: https://silexion.com
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the federal securities laws. All statements other than statements of historical fact contained in this communication, including statements regarding Silexion’s business strategy, ongoing preclinical studies evaluating SIL204 in pancreatic and colorectal cancer applications, potential expansion of development strategy, and the therapeutic potential of SIL204 across multiple cancer types, are forward-looking statements. These forward-looking statements are generally identified by terminology such as “may”, “should”, “could”, “might”, “plan”, “possible”, “project”, “strive”, “budget”, “forecast”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them, or similar terminology. Forward-looking statements involve a number of risks, uncertainties, and assumptions, and actual results or events may differ materially from those projected or implied by those statements. Important factors that could cause such differences include, but are not limited to: (i) Silexion’s ability to successfully complete preclinical studies and initiate clinical trials; (ii) Silexion’s strategy, future operations, financial position, projected costs, prospects, and plans; (iii) the impact of the regulatory environment and compliance complexities; (iv) expectations regarding future partnerships or other relationships with third parties; (v) Silexion’s future capital requirements and sources and uses of cash, including its ability to obtain additional capital; (vi) Silexion’s ability to maintain its Nasdaq listing; and (vii) other risks and uncertainties set forth in the documents filed or to be filed with the SEC by the Company, including the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 18, 2025. Silexion cautions you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth herein speak only as of the date they are made. Silexion undertakes no obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs, except as otherwise required by law.
Company Contact
Silexion Therapeutics Corp
Ms. Mirit Horenshtein Hadar, CFO
mirit@silexion.com
Capital Markets & IR Contact
Arx Capital Markets
North American Equities Desk
silexion@arxadvisory.com
![]()
