RetinalGenix Reports on its Science and Mission in Light of New AI-Driven Retinal Mapping Study
APOLLO BEACH, Fla., Jan. 14, 2026 (GLOBE NEWSWIRE) — RetinalGenix Technologies Inc. [OTCQB: RTGN] (“RetinalGenix” or the “Company”), a company developing ultra-high-resolution retinal imaging technology, today announced that a recent large‑scale Australian retinal mapping study, which used artificial intelligence to analyze approximately 50,000 retinal scans, reported that specific patterns of retinal thinning are associated with neurodegenerative and metabolic diseases such as dementia, diabetes, and multiple sclerosis. These findings support the broader field of oculomics and reinforce the premise that high‑quality retinal images, analyzed with advanced algorithms and integrated with genetic and clinical data, could become an important tool for earlier detection, risk stratification, and ongoing monitoring of disease. RetinalGenix’s core thesis—that combining high‑resolution imaging, genetics, and advanced analytics may unlock new insights into ocular and systemic health—is expected to position the Company to benefit as this field continues to mature.
RetinalGenix is developing a portfolio of non‑invasive imaging solutions, including a portable Retinal Imaging Screening Device and the RetinalCam remote monitoring system, designed for use without dilation and with real‑time connectivity to support longitudinal data collection. If successfully developed, validated, and cleared, these platforms could enable the generation of large‑scale, real‑world retinal datasets from pharmacies, clinics, urgent care centers, nursing homes, and potentially in‑home environments. Such a distributed imaging network could create opportunities for AI‑enhanced screening, earlier identification of disease signals, and more proactive patient management, while also providing valuable datasets for research and partnerships with payers, providers, and life sciences companies.
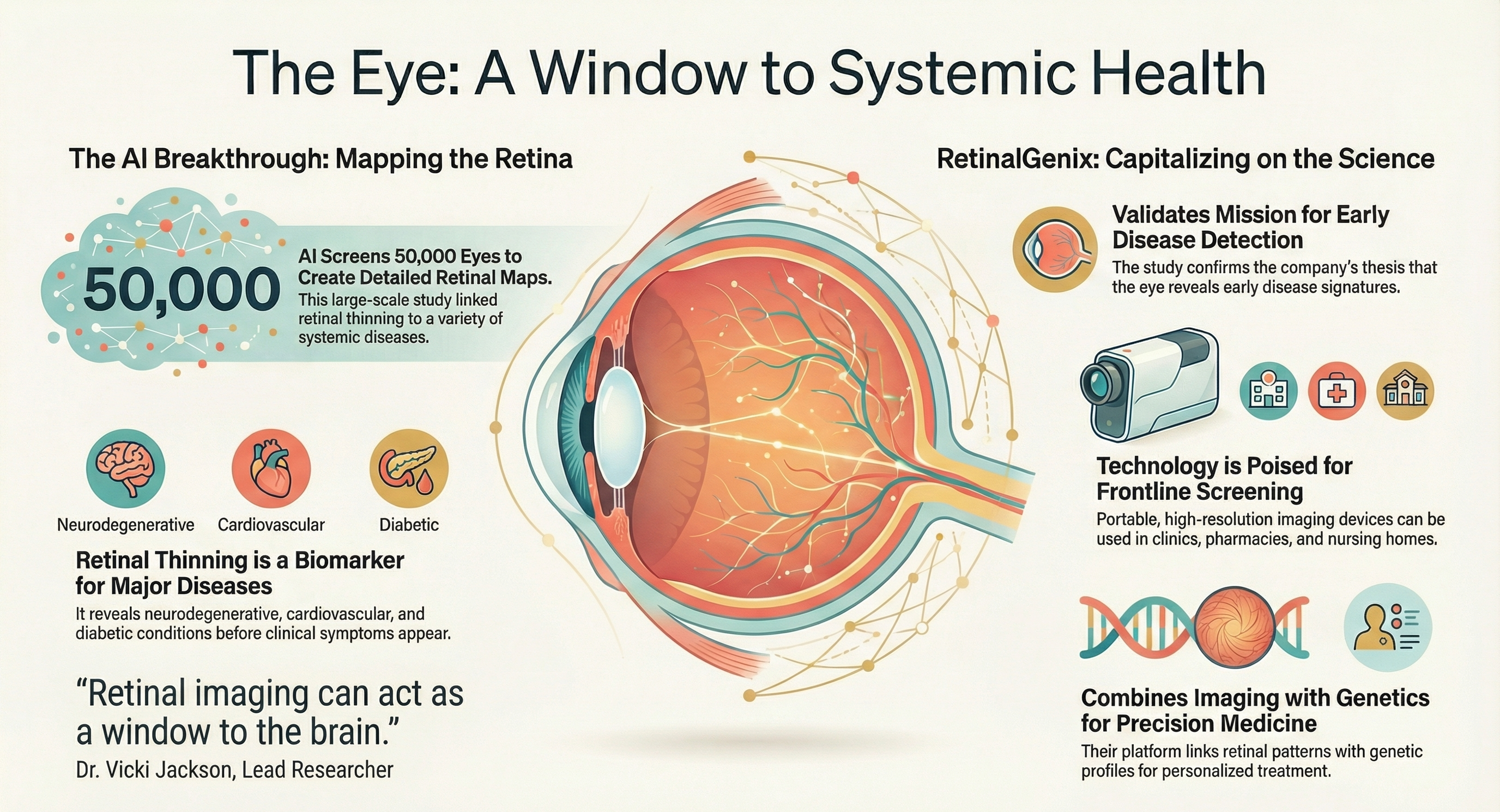
The same scientific trends that support the Australian study—convergence of imaging, AI, and genomics—also support RetinalGenix’s broader strategy, including programs in remote diagnostics, pharmacogenetic mapping, and therapeutics for conditions such as dry AMD and Alzheimer’s‑related dementia. Although further clinical studies, regulatory review, and commercial execution will be required, RetinalGenix believes it is well-positioned to participate in the emerging ecosystem where retinal imaging and AI‑driven oculomics could support more personalized, scalable, and preventive models of care.
RetinalGenix is advancing investigational, non-invasive, high-resolution retinal imaging technologies that are intended solely for informational purpose tools to capture retinal images that may indicate whether further clinical evaluation is warranted, rather than to provide any standalone diagnosis. Screened images and any associated findings must be reviewed and interpreted by qualified medical professionals, and the devices are currently investigational, “limited by Federal law to investigational use,” and are not cleared for the detection or diagnosis of systemic diseases, although the company’s strategic direction is aligned with emerging science suggesting that retinal imaging may play an important role in future precision-medicine screening paradigms.
About RetinalGenix Technologies Inc.
RetinalGenix is an ophthalmic research and development company seeking to revolutionize early disease detection and improve patient outcomes across multiple disease areas by integrating genetic screening, advanced imaging, and therapeutic development. Its proprietary High-Resolution Retinal Imaging and RetinalGenix DNA/RNA/GPS Pharmaco-Genetic Mapping™ technologies are designed to help prevent blindness by detecting initial physiological changes that could indicate future ocular and systemic diseases affecting neurodegenerative, cardiovascular, vascular, and metabolic systems, as well as diabetic conditions, Alzheimer’s disease, Complex Dementia, and Parkinson’s disease. RetinalGenix is also developing therapeutic drugs for dry age-related macular degeneration (dry AMD) and Alzheimer’s disease/dementia.
Safe Harbor Statement
This press release contains certain forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements are identified by the use of the words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential,” “project” and similar expressions that are intended to identify forward-looking statements and include statements regarding specific patterns of retinal thinning being associated with neurodegenerative and metabolic diseases such as dementia, diabetes, and multiple sclerosis, the findings supporting the broader field of oculomics and reinforcing the premise that high‑quality retinal images, analyzed with advanced algorithms and integrated with genetic and clinical data, could become an important tool for earlier detection, risk stratification, and ongoing monitoring of disease, combining high‑resolution imaging, genetics, and advanced analytics to unlock new insights into ocular and systemic health, the Company benefiting as the field continues to mature, developing a portfolio of non‑invasive imaging solutions, including a portable Retinal Imaging Screening Device and the RetinalCam remote monitoring system, designed for use without dilation and with real‑time connectivity to support longitudinal data collection, the platforms, if successfully developed, validated, and cleared, enabling the generation of large‑scale, real‑world retinal datasets from pharmacies, clinics, urgent care centers, nursing homes, and potentially in‑home environments, a distributed imaging network creating opportunities for AI‑enhanced screening, earlier identification of disease signals, and more proactive patient management, while also providing valuable datasets for research and partnerships with payers, providers, and life sciences companies, the convergence of imaging, AI, and genomics also supporting the Company’s broader strategy, including programs in remote diagnostics, pharmacogenetic mapping, and therapeutics for conditions such as dry AMD and Alzheimer’s‑related dementia, being well-positioned to participate in the emerging ecosystem where retinal imaging and AI‑driven oculomics could support more personalized, scalable, and preventive models of care, advancing investigational, non-invasive, high-resolution retinal imaging technologies that are intended solely for informational purpose tools to capture retinal images that may indicate whether further clinical evaluation is warranted, rather than to provide any standalone diagnosis, retinal imaging playing an important role in future precision-medicine screening paradigms, the study opening up the potential for using routine eye imaging to screen for and manage diseases, retinal imaging acting as a window to the brain, by detecting associations with neurological disorders like multiple sclerosis and many other conditions, the potential for retinal thickness as a diagnostic biomarker to aid in detecting and tracking the progression of numerous diseases. These forward-looking statements are based on management’s expectations and assumptions as of the date of this press release and are subject to a number of risks and uncertainties, many of which are difficult to predict, that could cause actual results to differ materially from current expectations and assumptions from those set forth or implied by any forward-looking statements. Important factors that could cause actual results to differ materially from current expectations include, among others, the Company’s ability to successfully complete research and further development and commercialization of the Company’s products, the timing, cost and uncertainty of obtaining regulatory approvals for the Company’s products, the Company’s ability to protect its intellectual property, and the risk factors described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024 and the Company’s subsequent filings with the SEC, including subsequent periodic reports on Forms 10-Q and 8-K. The information in this release is provided only as of the date of this release, and we undertake no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
Media Contacts:
RetinalGenix Technologies Inc.
Media and Investor Relations
ir@RetinalGenix.com
(800) 331-5446
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/3153d1b5-5946-4aaa-b8bf-bb744bccebcd
![]()
