Patent Allowance Granted for P-1 Molecule by US Patent and Trademark Office
Novel MDMA-Like Drug Could Be Used for Numerous Neurological and Psychiatric Indications
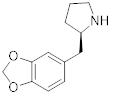
P-1 molecular structure
TORONTO, March 20, 2024 (GLOBE NEWSWIRE) — PharmAla Biotech Holdings Inc. (“PharmAla” or the “Company”) (CSE: MDMA) (OTC:MDXXF), a biotechnology company focused on the research, development, and manufacturing of LaNeo™ MDMA and novel derivatives of MDMA (MDXX class molecules), is thrilled to announce that allowance has been granted for the Composition of Matter of its PharmAla-1 (P-1) molecule by the US Patent and Trademark Office (USPTO). P-1 is an enantiomerically pure composition of ((R)-2-[(2H-1,3-benzodioxol-5-yl)methyl]pyrrolidine.
“In proof-of-concept rodent models at the laboratory of Prof. William Fantegrossi, P-1 showed a number of features which we believe to be extremely valuable in a therapeutic context,” said Dr. Harpreet Kaur, Vice President of Research at PharmAla Biotech. “The molecule has an excellent therapeutic window; it shows a strong pro-social signal at dosage levels far below racemic MDMA. We also believe it can induce neuroplasticity, like many drugs in the tryptamine class, by crossing the cell membrane and binding to serotonin receptors within the cell. And of course, like all the molecules we choose to develop, P-1 has an excellent safety profile with low abuse liability.”
The composition was identified through PharmAla’s computational chemistry program at the University of Windsor, made possible by a grant from the Ontario Centres of Innovation. P-1 was submitted to the USPTO through the Patent Prosecution Highway program, allowing for accelerated patent examination. As the molecule is ready for pre-clinical and clinical development, the Company has elected to change its designation to APA-001.
“We foresee that APA-001 could potentially be used in a number of indications. The company is currently assessing those indications which show the most promise,” said Nick Kadysh, Founding CEO, PharmAla Biotech. “The potential for a drug that could not only assist with psychological trauma, but also neurological damage, could find broad applicability in the treatment of conditions such as Traumatic Brain Injury (TBI) and stroke. In addition there is potential applicability for the fear disorders often discussed in the context of MDMA.”
About PharmAla
PharmAla Biotech Holdings Inc. (CSE: MDMA)(OTCQB:MDXXF) is a biotechnology company focused on the research, development, and manufacturing of MDXX class molecules, including MDMA. PharmAla was founded with a dual focus: alleviating the global backlog of generic, clinical-grade MDMA to enable clinical trials as well as commercial sales in selected jurisdictions, and to develop novel drugs in the same class. PharmAla is the only company currently provisioning clinical-grade MDMA for patient treatments outside of clinical trials. PharmAla’s research and development unit has completed proof-of-concept research into several IP families, including ALA-002, its lead drug candidate. PharmAla is a “regulatory first” organization, formed under the principle that true success in the psychedelics industry will only be achieved through excellent relationships with regulators.
For more information, please contact:
Nicholas Kadysh
Chief Executive Officer
PharmAla Biotech Holdings Inc.
Email: press@PharmAla.ca
Phone: 1-855-444-6362
Website: www.PharmAla.ca
Neither the Canadian Securities Exchange nor its Regulation Services Provider have reviewed or accept responsibility for the adequacy or accuracy of this release.
Cautionary Statement
This press release contains ‘forward-looking information’ within the meaning of applicable Canadian securities legislation. These statements relate to future events or future performance. The use of any of the words “could”, “intend”, “expect”, “believe”, “will”, “projected”, “estimated” and similar expressions and statements relating to matters that are not historical facts are intended to identify forward-looking information and are based on PharmAla’s current belief or assumptions as to the outcome and timing of such future events. Forward-looking information is based on reasonable assumptions that have been made by PharmAla at the date of the information and is subject to known and unknown risks, uncertainties, and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking information. The forward-looking information contained in this press release is made as of the date hereof, and PharmAla is not obligated to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as required by applicable securities laws. Factors that could cause actual results to differ materially from those anticipated in these forward-looking statements are described under the caption “Risk Factors” in PharmAla’s management’s discussion and analysis which is available on PharmAla’s profile at www.sedar.com.
This news release does not constitute an offer to sell or the solicitation of an offer to buy, and shall not constitute an offer, solicitation or sale in any state, province, territory or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state, province, territory or jurisdiction.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/351506ca-33a3-4545-a1a7-6d44accfaa0e
![]()
