Breaking Ground in Prostate Cancer: BriaCell Announces Lead Prostate Cancer Candidate Bria-Pros+, Initiates GMP Manufacturing
Bria-Pros+ Activates Multiple Immune Cell Types
Bria-Pros+ Activates Immune Cells to Kill Cancer Cells
- Bria-Pros+ is based on BriaCell’s next generation personalized immunotherapy platform
- Bria-Pros+ is capable of activating multiple types of immune cells, including helper T cells, cytotoxic (killer) T cells and natural killer cells
- Initiation of GMP manufacturing represents a major milestone for BriaCell’s off-the-shelf personalized cellular cancer vaccines
PHILADELPHIA and VANCOUVER, British Columbia, Feb. 06, 2024 (GLOBE NEWSWIRE) — BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company developing novel immunotherapies to transform cancer care, is pleased to announce the initiation of Good Manufacturing Practice (GMP) manufacturing of its lead candidate for treating prostate cancer, Bria-Pros+. GMP manufacturing of Bria-Pros+ will provide clinical supplies for planned clinical trial use.
“Building upon the compelling pre-clinical proof-of-concept data presented at the Society for the Immunotherapy of Cancer (SITC) meeting in 2023, BriaCell’s proprietary platform technology holds significant potential to benefit patients across a spectrum of cancers. We are enthusiastic about commencing GMP manufacturing for our prostate cancer clinical candidate, continuing our efforts at transforming targeted cancer therapy via the introduction of potent personalized cellular cancer vaccines,” stated Dr. William V. Williams, BriaCell’s President and CEO. “The successful development of this first candidate showcases our capacity to create novel cancer immunotherapies and advance them towards clinical application. We eagerly anticipate completing Investigational New Drug (IND)-enabling studies during 2024 with Bria-Pros+.”
Recently presented at SITC 2023, the pre-clinical proof-of-concept data demonstrated both feasibility and efficacy of BriaCell’s platform of cellular cancer vaccines overall, with specific emphasis on Bria-Pros+. BriaCell genetically engineers cancer cell lines to produce cytokines and co-stimulatory factors that significantly increase immune stimulation compared to the unmodified (parent) cancer cell lines. These cell lines also express patient-specific Human leukocyte antigens (HLA) alleles and potentially provide personalized off the shelf treatment.
In the realm of cancer immunotherapy, the objective is to restore the body’s natural anti-tumor immunity. Despite notable progress, current approaches often fall short of achieving curative outcomes, primarily because they target specific immune processes, resulting in only partial restoration of the body’s inherent anti-cancer immunity.
An optimal cancer immunotherapy should initiate or reinstate a persistent anti-tumor immune response via both complementary and diverse mechanisms resulting in a self-sustaining cycle of cancer immunity by both the innate and adaptive immune responses. The data highlighted at the SITC meeting demonstrated that Bria-Pros+ could effectively activate the natural immune response against tumor cells by both expressing cancer antigens, and by modulating the activity of innate and adaptive immune cells. These include helper T cells (CD4+), cytotoxic (killer) T cells (CD8+), and natural killer cells (both Classical NK cells and NKT cells). Data previously presented at the SITC meeting is reproduced here in a different format.
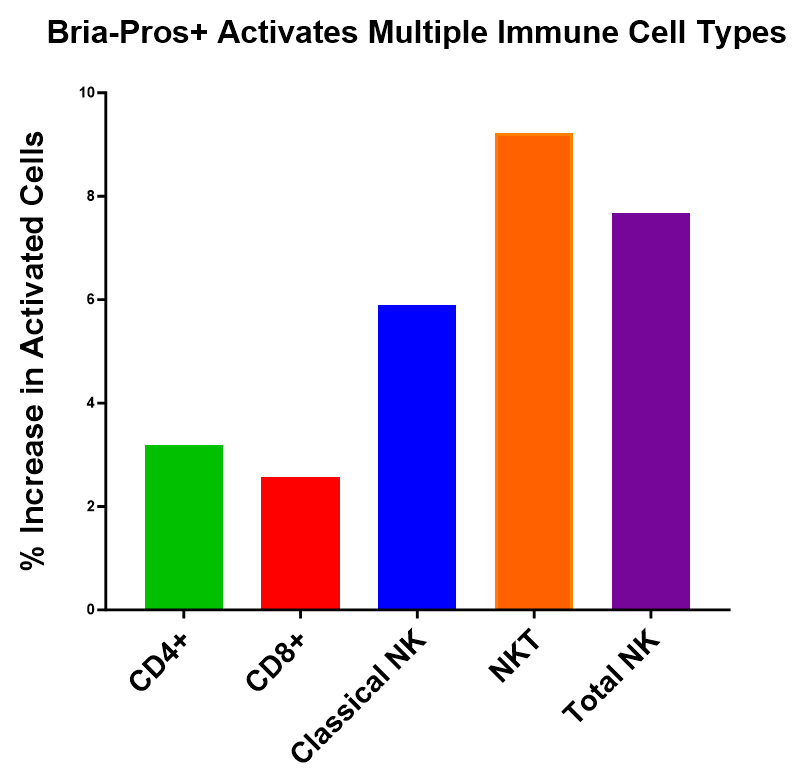
Figure 1 shows that Bria-Pros+ activates a range of immune cells in a modified mixed lymphocyte reaction. After 48 hours of co-culture, immune cells were analyzed. CD4+ & CD8+ T cells, NK-T and Classical NK cells were activated as shown here as the % increase in activated cells following Bria-Pros+ stimulation compared with parent cell stimulation.
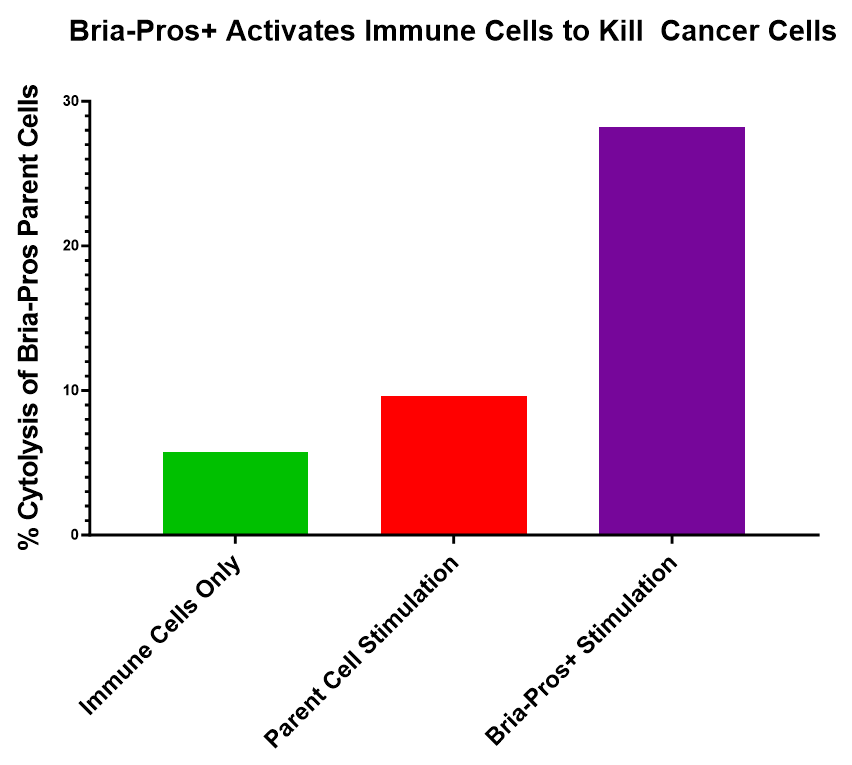
Figure 2 shows that Bria-Pros+ cells can stimulate immune cells to kill prostate cancer cells. Engineered to express co-stimulatory and immuno-modulatory cytokines, Bria-Pros+ cells enhance the ability of immune cells to kill tumor cells.
In summary, our data suggests significantly stronger anti-tumor activity for Bria-Pros+ vs that of the parent cells, making it an ideal lead clinical candidate for our upcoming clinical studies in prostate cancer.
According to 2024 Cancer Facts & Figures , prostate cancer is projected to be the most common cancer among men in 2024. With 299,010 new cases estimated to be diagnosed in 2024 and 35,250 projected deaths from prostate cancer in 2024, prostate cancer is expected to be the second leading cause of cancer death among men in 2024. Current treatments for metastatic prostate cancer include immunotherapy, hormone therapy, chemotherapy and targeted treatments. Novel approaches are needed for advanced prostate cancer.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those about: GMP manufacturing of Bria-Pros+ providing clinical supplies for planned clinical trial use; BriaCell’s proprietary platform technology benefitting patients across a spectrum of cancers; the commencement of GMP manufacturing; BriaCell’s capacity to transform targeted cancer therapy via the introduction of personalized cellular cancer vaccines; BriaCell’s capacity to create novel cancer immunotherapies and advance them towards clinical application; completing IND-enabling studies during 2024 with Bria-Pros+; Bria-Pros+ effectively activating the natural immune response against tumor cells; and BriaPros+ ability to stimulate immune cells to kill prostate cancer cells, are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties” in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company’s profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
info@briacell.com
Media Relations:
Jules Abraham
CORE IR
julesa@coreir.com
Investor Relations Contact:
CORE IR
investors@briacell.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/fd8a1c25-2dd0-48c8-9250-8ebfd55bd617
https://www.globenewswire.com/NewsRoom/AttachmentNg/94d150b3-b5f5-40e6-92fb-cbdb75a4f66c
![]()
