Annovis Reports Peer-Reviewed Publication Highlighting Pharmacokinetics of Novel Crystal Buntanetap
MALVERN, Pa., Sept. 16, 2025 (GLOBE NEWSWIRE) — Annovis Bio, Inc. (NYSE: ANVS) (“Annovis” or the “Company”), a late-stage clinical drug platform company pioneering transformative therapies for neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), today announced the publication of a new article in a peer-reviewed journal Biomolecules, which describes the pharmacokinetic (PK) profile of a new crystal form of buntanetap and compares it to the old form across several studies including mice, dogs, and humans.
The original anhydrous form of buntanetap has been used in all preclinical and clinical studies to date. Recently, the Annovis team identified a new dihydrate crystal, which incorporates two moles of water in its structure and offers greater stability. This publication examines how the new crystal form behaves in the body following oral administration, focusing on absorption and clearance. The findings confirm that crystal buntanetap preserves the PK profile and metabolism of the original form, which are essential for the drug’s therapeutic efficacy.
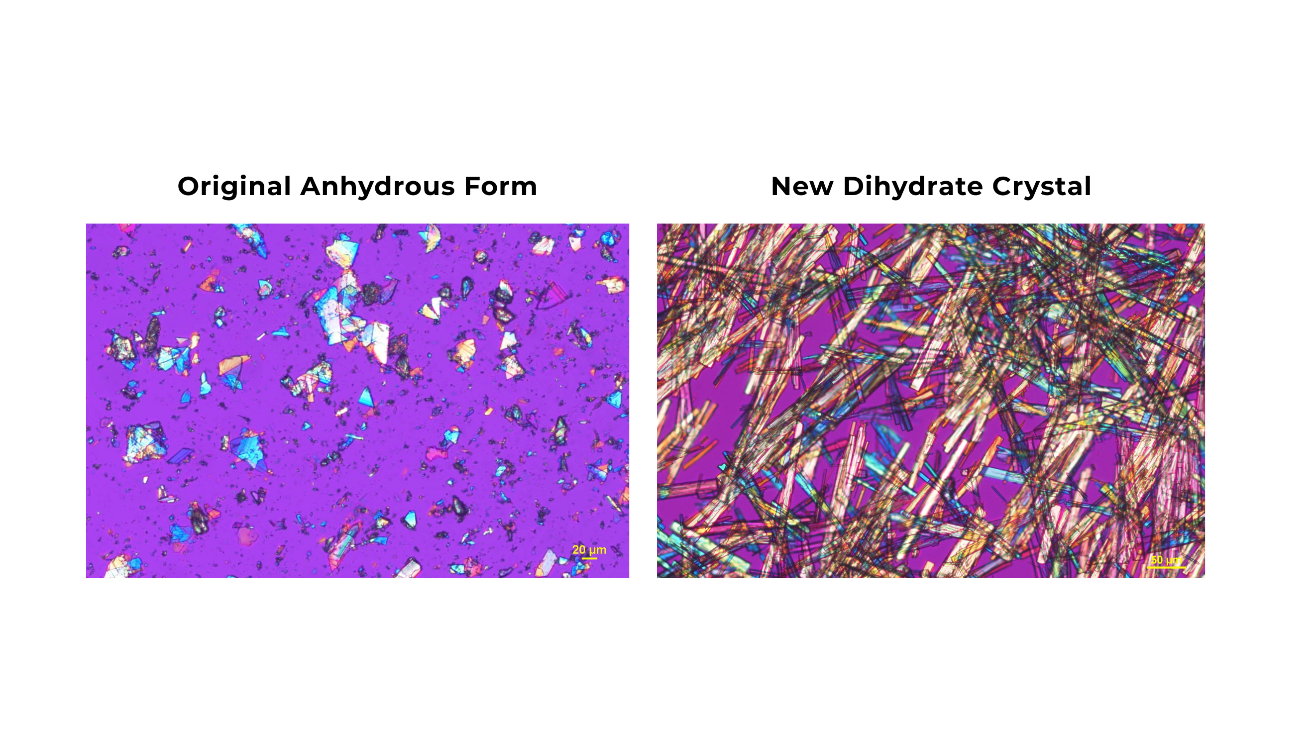
“We have highlighted the new form of buntanetap in prior presentations, but this open-access publication presents the data in full to a broader audience. It not only validates the use of crystal buntanetap in clinical trials but also reinforces the consistency and reliability of our lead asset,” said Alexander Morin, Ph.D., Director of Strategic Communications. “PK is central to understanding how a drug works, and we remain committed to rigorous analysis of buntanetap to ensure the most beneficial outcomes for patients.”
The new crystal buntanetap is currently being used in the pivotal Phase 3 clinical trial (NCT06709014) in patients with early AD, which is actively enrolling participants across the U.S. The study is designed to deliver two key readouts: symptomatic (Fall 2026) and disease-modifying (Fall 2027). Moreover, this new form extends the intellectual property protection of the Company’s lead compound into the 2040s, covering its mechanism of action, therapeutic use, and combination with other drugs.
The publication can be accessed online via the LINK or found in the Publications library on Annovis’ website.
About Annovis
Headquartered in Malvern, Pennsylvania, Annovis is dedicated to addressing neurodegeneration in diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). The Company is committed to developing innovative therapies that improve patient outcomes and quality of life. For more information, visit www.annovisbio.com and follow us on LinkedIn, YouTube, and X.
Investor Alerts
Interested investors and shareholders are encouraged to sign up for press releases and industry updates by registering for email alerts at https://www.annovisbio.com/email-alerts.
Forward-Looking Statements
This press release contains forward-looking statements under the Securities Act of 1933 and the Securities Exchange Act of 1934, as amended. Actual results may differ due to various risks and uncertainties, including those outlined in the Company’s SEC filings under “Risk Factors” in its Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. The Company undertakes no obligation to update forward-looking statements except as required by law.
Contact Information:
Annovis Bio Inc.
101 Lindenwood Drive
Suite 225
Malvern, PA 19355
www.annovisbio.com
Investor Contact:
Alexander Morin, Ph.D.
Director, Strategic Communications
Annovis Bio
ir@annovisbio.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b56f10e2-7690-4b2a-85bc-cb17a3e3bd43
![]()
